GAL-3
Genetic Screening
Empower Your Patients with a Pathway to Potential Dementia Reversal
GAL-3
Genetic Screening
Empower Your Patients with a Pathway to Potential Dementia Reversal





833-363-6226
Support (8:00 AM - 7:00 PM PDT)
What is the GAL-3 Genetic Screening Test?
Supporting providers and investigators providing TB006 under Expanded Patient Access
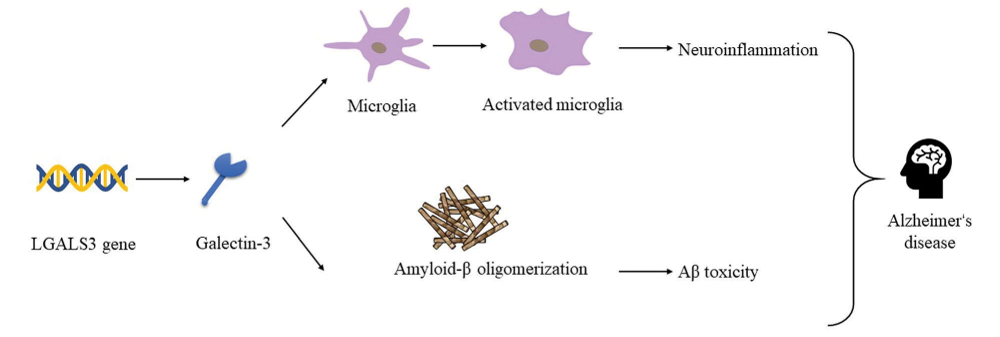
The GAL-3 Genetic Screening Test provides your patients with key information to confidently determine if they are genetically suited for TB006 therapy. This monoclonal antibody therapy has shown promise in potentially reversing dementia. By targeting the LGALS3 gene, which regulates galectin-3, this test delivers crucial insights to guide personalized treatment decisions.
A simple, non-invasive genetic test that analyzes your patient’s DNA:
- Focuses on the LGALS3 gene, which regulates galectin-3, a protein implicated in dementia
- Determines if your patient possesses the genetic profile that may respond to TB006 treatment
TB006 Screening is Easy

Register your clinic
Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Register your patients with the barcode ID on the collection device
Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Affordable Health Packages
Lorem ipsum dolor sit amet, consectetur adipiscing elit.
Easy as 1-2-3
1
Register & Setup your clinic
2
Register your patients with the barcode ID on the collection device
3
Collect the sample & mail in the prepaid priority shipping envelope
GAL-3 Collection Kits
Collection kits
GAL-3 DNA Collection Kit (Bundle)
5 Complete kit including prepaid priority shipping to our lab.

we’re here to Answer all your questions
Information about genetic testing for TB006 therapy.
What is the GAL-3 Function DNA Report?
The GAL-3 Function DNA Report is a simple, non-invasive genetic test that analyzes a patient’s DNA using a cheek swab. It focuses on variations in the LGALS3 gene, which regulates Galectin-3, a protein associated with inflammation, neurodegeneration, and cognitive decline—including dementia-related conditions.
What is the clinical purpose of the GAL-3 DNA test?
This test helps determine whether your patient carries a specific genetic profile that blocks TB006—a monoclonal antibody therapy—rendering it ineffective in approximately 1 out of every 25 treated patients. Identifying this profile in advance allows for more informed treatment decisions and personalized care strategies.
Is this test diagnostic or predictive?
This is a Research Use Only (RUO) genetic report and is not intended to diagnose disease. It offers predictive insights based on known gene-environmental interactions and current literature, which can be used to inform clinical decision-making.
How is the DNA collected?
The test uses a non-invasive cheek swab, which can be completed in minutes in-office or at home with clear instructions provided.
How long does it take to receive results?
Once the sample reaches our certified lab, results typically take 10–14 business days.
How will I receive the results?
Test results are delivered securely through the provider portal or email. You will receive a notification once your patient’s report is ready to view and download.
How do I register a patient for testing?
Visit tb006test.com/registerpatient and follow the prompts to securely register your patient and assign their sample kit using the barcode provided.
How do I order more DNA test kits for my clinic?
Use this link to order additional collection kits. These are collection kits only. You will only pay the lab fee when you later order the actual test.
Do I need a test kit to register a patient?
Yes. You will need the barcode or kit ID provided with the DNA swab kit. This links the patient to their unique test and enables proper lab processing.
Is patient data secure?
Yes. All data is protected with advanced encryption and complies with HIPAA and CLIA standards. No data is shared or sold without explicit consent.
Can patients request deletion of their data?
Absolutely. Patients can request full deletion of their personal and genetic information at any time by contacting support@endodna.com.

